Project: Bcl2 interactions in the membrane: a matter of life or death [PID2023-152568NB-I00]
Spanish Ministry of Science, Innovation and Universities
Summary
Regulated or programmed cell death consists of a set of molecular pathways responsible for maintaining the homeostasis of an organism. Given their importance, these molecular pathways are highly regulated and their dysregulation is associated with the onset of several diseases, from neurodegenerative disorders to viral pathologies or cancer.
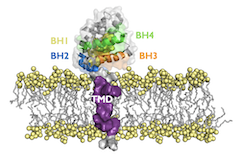
Apoptosis, the main mechanism of controlled cell death, is an evolutionarily conserved cellular process that occurs in response to various physiological and pathological situations. There are two main pathways for the activation of apoptosis: the extrinsic and the intrinsic pathways. The intrinsic pathway is primarily regulated by the Bcl2 protein family. Members of the Bcl2 protein family are characterized by the presence of sequence homology domains, known as BH domains and in most cases a carboxy-terminal transmembrane domain (TMD) that anchors the protein to the mitochrondria outer membrane. Various competing models describe how Bcl2 family proteins interact with each other to control apoptosis. Regardless of the model, it is well accepted that the extramembrane BH domains play a key role in the Bcl2 family protein-protein interactions (PPI). However, our team has been at the forefront of research demonstrating that some Bcl2 interact through their TMDs that collaborate with cytosolic regions in the modulation of cell death. Considering the TMD as an active player represents a major shift in the current paradigm and a significant advance in our understanding of the control of apoptosis. These findings have allowed us to identify new points of therapeutic intervention that could be crucial in the development of therapies for the treatment of diseases such as cancer.
The current proposal aims to unravel the Bcl2 protein family interactome with special emphasis on the role of TMDs in these interactions and on the control of apoptosis as potential therapeutic targets. Precisely we will:
- Explore the intramembrane interaction network of the Bcl2 family: We first propose to extend our study of TMD-TMD interactions to a larger set of Bcl2 protein family members. Establishing the intramembrane interaction network of Bcl2 proteins and their molecular details will help us to understand how apoptosis is regulated and will facilitate the development of new molecules that regulate this key process.
- Define a global interaction map of the Bcl2 family: We propose using an omic approach to explore PPIs involving selected members of the Bcl2 protein family, under both resting and apoptotic conditions. We will focus on identifying potential chaperones required for the targeting and insertion of Bcl2 members into the mitochondrial membrane. Once in the mitochondria, we propose to further characterize some critical aspects, such as the role of Bcl2s TMD in the interactions or the dynamics of the interactions as apoptosis progresses.
- Preclinical development of D1: We have previously identified a transmembrane peptide, D1, able to inhibit BclxL, a carcinogenic member of the Bcl2 family. D1 represents a new type of Bcl-xL inhibitor with a promising anti-cancer profile. However, much work needs to be done to develop an effective cancer treatment. With the current proposal, we pretend to take the first steps in this long path which involve: defining the therapeutic scope of D1, establishing a formulation compatible with pre-clinical assays, and evaluating its toxicity and efficacy in a suitable preclinical model.